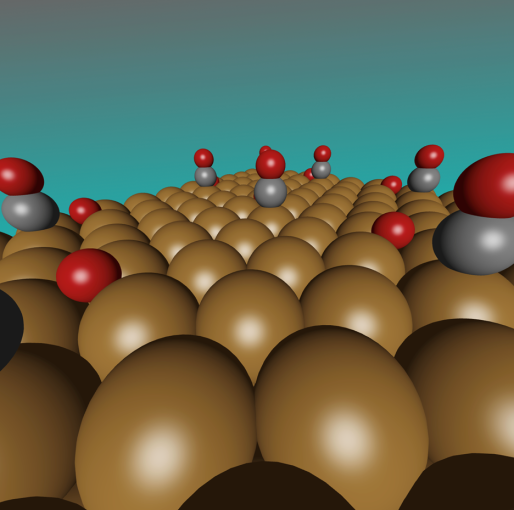


In the previous report, it was informed that a computer simulation using Python programming has successfully been developed to investigate the reaction of reducing vehicle exhaust emissions to harmless gases, specifically converting NO into N₂. In this study, copper was used as the main catalyst for the reaction. Copper is used in this study because it is more affordable and readily available compared to ‘premium’ materials like platinum, palladium, or rhodium, which are typically used in catalytic converters. Eventhough these metals are effective, they are very expensive and rare. In contrast, copper is much more accessible and widely available.
This research supports global health in line with SDG 3: Good Health and Well-being, which aims to reduce diseases and deaths caused by hazardous chemicals and pollution. Air pollution poses a significant health risk, particularly in urban areas, where it can lead to respiratory and heart problems. One of the biggest challenges in reducing NO emissions is managing emissions during the cold start phase when a car’s engine is first turned on. During this phase, traditional catalytic converters do not operate efficiently. This study found that copper can effectively reduce NO emissions even at low temperatures, making it a potential solution to this issue. By incorporating copper into catalytic converters, vehicles can start cleaner right from the beginning.
Although copper shows great potential, further research is needed to enhance its performance at higher temperatures. Combining it with other materials or modifying its properties could increase its effectiveness across various engine temperature conditions. Real-world testing is also crucial to ensure that these findings are applicable to everyday use.
So, what does this mean for you? This research could help lower the cost of environmentally friendly vehicles, making them more accessible. Additionally, the findings recommend copper as a catalytic additive for converters, promoting cleaner air and reducing health risks. With these innovations, production costs could become more affordable and environmentally friendly, supporting global efforts to improve public health.
The research covers several points of the Sustainable Development Goals (SDGs), particularly SDG 3: Good Health and Well-Being, which aims to improve global health by reducing the risk of diseases and deaths caused by pollution and harmful chemicals. This study focuses on reducing vehicle exhaust emissions, specifically converting NO gas into harmless gases, which can help address air pollution—a major health risk in urban areas. Additionally, the research also contributes to SDG 12: Responsible Consumption and Production by proposing the use of more affordable and accessible copper as an alternative to conventional, expensive catalytic materials. This could lower the cost of eco-friendly vehicles and improve access to clean technologies. The innovation has the potential to enhance air quality and support global efforts toward better public health.
